Children
Childhood cancer survivors often suffer from late effects. Radiotherapy is an important part of the multidisciplinary treatment of pediatric cancers. Its use is always a balance of potential benefits and possible side effects. In this project we will register side effects after radiotherapy in children in the already established Danish Childhood Cancer Registry. The side effects will then be correlated to the radiation doses in the irradiated tissues. This database will permit us to learn more about the effect of radiotherapy in children, help to prevent and treat toxicities and increase the awareness of the risk of late effects. The data will be the basis for establishing guidelines for follow-up and for future research in the field. The project will be established in close collaboration with other European groups.

Aim
To establish a prospective database of all pediatric patients treated with radiation for the evaluation of late effects after radiation in childhood and adolescence. Simultaneously a retrospective data collection from children and adolescents irradiated within the last decade will be established. The collected information will increase the knowledge about normal tissue tolerances in pediatric patients, increase awareness about late effects, help to prevent and treat toxicities and serve as a basis for national and international research collaborations in the field. First projects will be based on the two cohorts:
- Identification, prediction and reduction of treatment-induced brain tissue changes following photon vs proton radiotherapy in children with CNS cancer (MRI Project, PI Oscar Casares Magaz)
- Testing of potential radiobiological biomarkers for prediction of acute and early late toxicity in a pediatric population (with WP 1) (Radiobiology Project, PI Yasmin Lassen)
- The impact on quality of life of receiving proton radiotherapy in a foreign country or in the home country (Quality of Life Project, PI Michael Thude Callesen)
Background
Childhood cancer is rare with approximately 200 new cases in Denmark every year. Currently, 80% of children in developed countries survive their cancer. Radiotherapy is together with surgery and systemic antineoplastic therapies one of the main treatment components for many solid tumours in children. Multiple studies have shown that pediatric radiotherapy leads to non-neglectable late effects1 that can emerge short time but also after a latency of decades after the radiotherapy treatment and that can be devastating for the patient’s quality of survival. The main literature on radiation-induced late effects is often based on retrospective studies and only phantom estimated reconstructions of the actual given dose. Often outdated radiotherapy techniques have been used. Recently the literature shows a trend to less toxicity2 in general after combined oncological treatment from the modern era. In the last decade proton radiotherapy has been more often employed to treat pediatric patients because of the favorable dose distribution that can often spare normal tissues to a higher extent than traditional photon radiotherapy. First studies concerning toxicity after proton treatment are emerging.3
Dose-volume effects in pediatric patients are not very well established. The phantom based retrospective studies are not very precise and describe outdated field arrangements and technologies. Data from adult studies might not describe the situation of irradiating a tumour in growing and immature normal tissue like in children. Therefore prospective registration of dose-volume data in relation to the development of toxicities after photon and proton radiotherapy is important. Data will permit to establish pediatric normal tissue tolerances, enabling better estimates of risks of late effects and potentially better prevention. It will also permit to establish guidelines for follow-up of children and adolescents treated with radiation.
Methods
Toxicity data after radiotherapy in childhood and in adolescents will be added to the Danish Childhood Cancer Registry. Radiation parameters will be saved in the National Doseplanbank as well as follow up images. Patient-reported outcome information, acute and late toxicity parameters will be systematically collected by radiation- and pediatric oncologists. An international workshop with experts in the fields will be organized, to ensure that the registry will be usable in an international context to make larger research collaborations possible.
A registry containing retrospective data of children treated in the last 10 years with radiation in Denmark will also be established. This data will be collected from the four Danish centers treating Pediatric Cancer by dedicated research assistants.
Expected results
To establish a comprehensive, prospective database about the quality of life, acute and late toxicities of children treated with radiotherapy in Denmark. To establish normal tissue tolerance models for pediatric radiotherapy. To establish a retrospective cohort of children treated with radiotherapy in the last ten years. First research projects will be started based on the two cohorts. Future projects will be designed.
The study is summarized in figure 1 below.
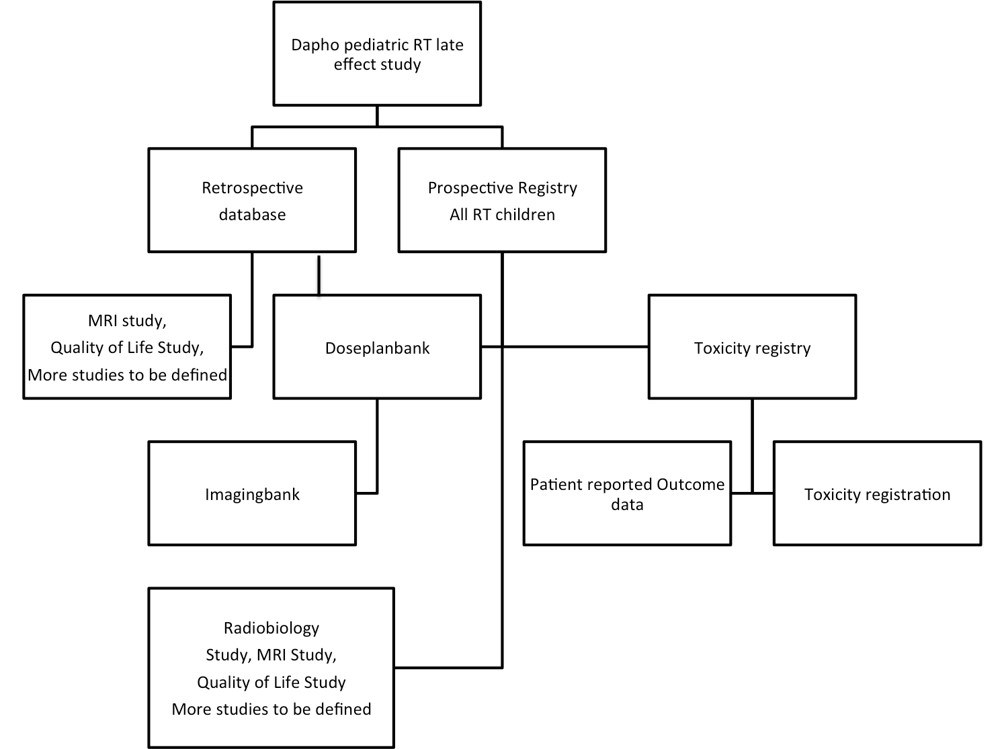
Figure 1. Flowchart of the study
Impact/relevance/ethics
The overall goal of these cohorts is to provide every childhood cancer survivor with better care and better long-term health so that they reach their full potential, and to the degree possible, enjoy the same quality of life and opportunities as their peers. Projects will apply with all ethical standards.
Clinical trials
- HARMONIC-RT, ClinicalTrials.gov identifier: NCT04746729
-
Akmal Safwat
Overlæge
Aarhus University Hospital![]()
-
Michael Callesen
MD, PhD
Odense University Hospital![]()
-
Maja Vestmø Maraldo
Physician, PhD
Rigshospitalet, Copenhagen![]()
-
Laura Ann Rechner
PhD student
Rigshospitalet, Copenhagen![]()
-
Yasmin Lassen
Senior Consultant
Aarhus University Hospital -
Daniella Østergaard
MD, PhD student
Rigshospitalet, Copenhagen![]()




